Publications
10) “Strategic Advances in the Total Syntheses of Opioids: Codeine, Morphine, and Related Alkaloids”,
Mondal, A.; Mondal, A.; Dutta S. and Bisai A.*
Asian J. Org. Chem., 2025, e00333. (https://doi.org/10.1002/ajoc.202500333)

9) “Catalytic Asymmetric Total Synthesis of Naturally Occurring Amaryllidaceae Alkaloid, (+)-11-Hydroxyvittatine”,
Mondal, A.; Mondal, A.; Roy, T. and Bisai A.*
Org. Lett. 2025, 27, 25, 6878–6883. (https://doi.org/10.1021/acs.orglett.5c02057)


8) “Total Synthesis of (+)-Dixiamycin C via Ni(II)-Photoredox Aryl Amination”,
Niyogi S.; Mondal A.; Nandy, M.; Khatua, A.; Bisai, A.*
Org. Lett., 2024, 26, 41, 8643–8647 (https://doi.org/10.1021/acs.orglett.4c02436)

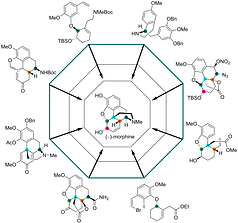
7) “Concise Total Synthesis of (−)-Codeine”,
Mondal A.; Pal S.; Khatua A.; Mondal A. and Bisai, A.*
J. Org. Chem. 2024, 89, 17, 12485–12497 (https://doi.org/10.1021/acs.joc.4c01452)
-
Highlighted in SynFacts by Prof. Dirk Trauner, Synfacts 2024; 20(11): 1209.
-
Was part of the most read article on the JOC for the month September 2024.

6) “Biomimetic Total Synthesis of the Reported Structure of (+)-Selaginedorffone B”
Kundu, S.; Jana, D.; Mandal, N.; Mondal, A.; Murmu, R.; Roy, N. K.; Datta, A.* and Bisai, A.*
Chem. Sci., 2024, 15, 14946-14953 (https://doi.org/10.1039/D4SC04103H)

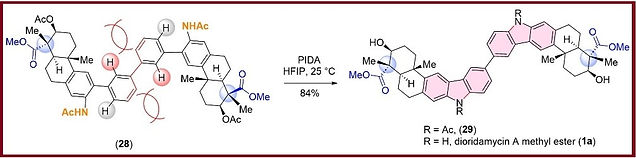
5) “Highly Regioselective Oxidative Csp2-H Amination for Indolosesquiterpene Alkaloids: Total Synthesis of (+)-Dioridamycin”
Nandi R.; Niyogi S.; Kundu, S.; Mondal A.; Roy, N.K. and Bisai, A.*
Organic Chemistry Frontiers, 2025, 12, 928-935. (https://doi.org/10.1039/D4QO01773K)

4) “Highly Chemoselective Oxidative Dimerization of Indolosesquiterpene Alkaloids: Biomimetic Approach to Dixiamycin”,
Munda, M.;§ Mondal, A.;§ Roy, N. K.; Murmu, R.; Niyogi, S.; and and Bisai, A.* (§ both author contributed equally)
Chem. Sci., 2024, 15, 9164-9172. (https://doi.org/10.1039/D4SC01396D)

3) “Concise Total Syntheses of Bis(cyclotryptamine) Alkaloids via Thio-Urea Catalyzed One Pot Sequential Michael Addition”,
Khatua, A.; Shyamal, P.; Pal, S.; Mondal, A. and Bisai A.*
Chem. Comm. 2022, 58, 3929 (https://doi.org/10.1039/D2CC01008A)

2) “Asymmetric Total Syntheses of (−)-Dihydromaritidine and (−)-Oxomaritidine”,
Yadav, A.; Majumder, S.; Das, M. K.; Mondal, A. and Bisai A.*
Synlett, 2022, 34, 07, 858-862. (https://doi.org/10.1055/a-1912-2378)

1) "Total Syntheses of (+)- and (−)-Crinane via Pd(0)-Catalyzed Deacylative Allylation"
Das, M. K.; Yadav, A.; Majumder, S.; Mondal, A. and Bisai A.*
Tetrahedron, 2021, 82, 131928. (https://doi.org/10.1016/j.tet.2021.131928)







